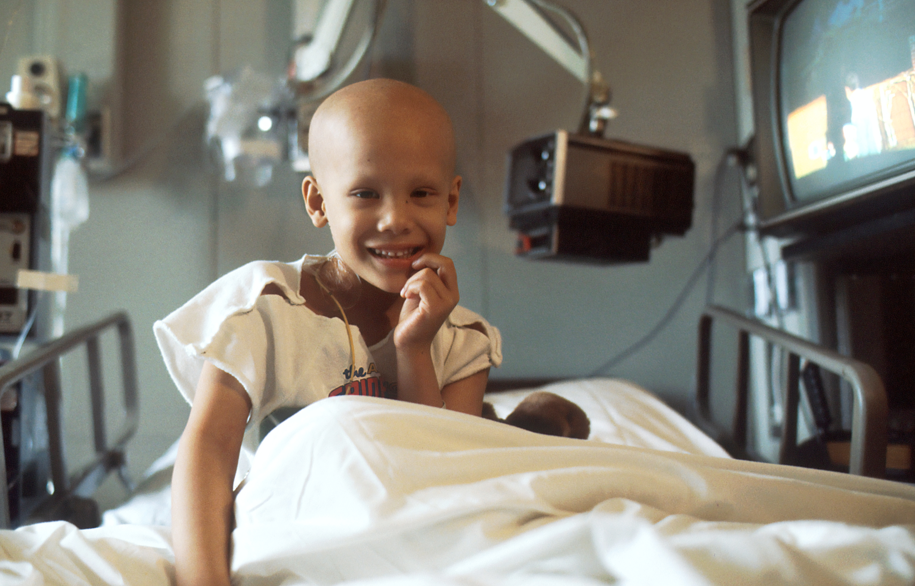
Ependymoma is the third most common type of brain tumour in children.
Currently the only effective treatments for these tumours are surgery and radiation.
When combined, these two approaches are successful in about 70% of cases. However, for one-in-three children the tumour will grow back and when it does there is no other effective treatment available, at which point, tragically this cancer is essentially incurable.
The treatments for ependymoma have not changed in any significant way for decades, which is why we need a new approach. That is where philanthropy comes in!
Thanks to the commitment of just over $1.2 million dollars over five years by the amazing Kids with Cancer Foundation, a world first drug trial has now begun at Sydney Children’s Hospital, Randwick under the leadership of Professor David Zeigler, with the first child recruited into the study in August of this year.
The Deflexifol at Relapse Trial, proudly supported by Kids with Cancer Foundation (DART) is a world first study investigating Deflexifol® as a promising new treatment for ependymoma and other brain cancers in children.
Deflexifol® is a new Australian-developed co-formulation of a chemotherapy drug called 5-FU and leucovorin.
5-FU has been effective in the past against ependymoma, but until now it has not been possible to deliver a therapeutic concentration of the drug into a child’s brain.
Combining these two drugs produces a potentially more potent and effective drug that should also produce fewer side effects, holding the promise of hope for families where previously there was none.
Learn more about how DART will help ependymoma patients like Charlotte.
5-FU has shown to be active against ependymoma but until now, it hasn’t been possible to get high enough concentrations of 5-FU into the brain. The Deflexifol® co-formulation has potential to be more potent and deliver higher doses of 5-FU, as leucovorin increases the drug’s potency. The treatment should also produce less side effects, holding promise for a more effective treatment for this complex disease. There is cause for optimism as there have been promising results already in an ongoing trial in adults.
Though, a trial of this drug in adult patients has been running for several years, those results are not directly applicable to paediatric patients. Chemotherapy dosage size and frequency are vitally important, and children differ physically in many ways to adults, meaning that what works in the adult population will not necessarily work in children. Chemotherapy drugs are also highly toxic so accurate dosage is vital for patient safety and to minimise long term side effects.
If the DART trial is a success, it will offer hope to families where previously there was none.